Finding Your Market: The Significance of an Effective Go-To-Market Strategy
What are your thoughts when purchasing a product? Do you think, Will I Need This? What is This For? What’s the Difference Between This and Another Product? How Will This Benefit My Patients? These, and other frequently asked questions, are common examples of Go to Market Strategy within the Medical Device industry. More importantly, these are examples of an effective Go To Market Strategy, crucial for launching a product into your intended market. This article will delve into the elements which comprise an effective GTM Strategy, and how to avoid market setbacks.
To understand how an effective GTM Strategy aids a product’s market launch, we must first examine what such a strategy looks like. Every effective strategy begins with product messaging. Simply put, it’s the message your audience is sent regarding your product. In turn, consumer’s perceptions are influenced by the messaging you put out1. A key framework to optimize your messaging is communicating what your product is, its intended use, your target audience, and how your product differentiates from the competition2. In other words, you are stating the specific goals your device will accomplish. This message utilizes key elements that often comprise an effective GTM strategy: Value Proposition, Competitive Analysis, Target Market, and Distribution Channels3.

Value Proposition: What is the market need for your device, and how will your device meet it? Essentially, what problem are you attempting to solve? Your Value Proposition takes into account your device’s potential benefits on the patient/provider experience, pricing differences compared to equivalent devices on the market, and how your device could become a “first in-class” solution with an innovative approach3.
Target Market: What is your target audience? How old are they? Where in the buying cycle are they? Are there any obstacles they may face with purchasing3? By understanding your ideal consumer’s mindset, you can tailor your GTM Strategy accordingly, and maximize your market outreach. In other words, these insights allow you to tailor specific messaging to specific consumer groups3.
Competitive Intelligence:
Who are your competitors, and how well are they doing? What is their device, and what are their selling tactics3? An understanding of your competition and their potential reactions to your market launch are crucial for an effective GTM Strategy. This analysis could potentially reveal a marking tactic which could be worth adopting, and grants you the ability to prepare responses to how competitor’s may react3.
Distribution Channels:
How will your product make its way to consumers? A crucial and sometimes forgotten step, distribution must be defined for consumers and reflected in your messaging. Will you handle the device’s distribution, or will it be coordinated by an outside vendor3? Where would consumers be able to acquire your device?
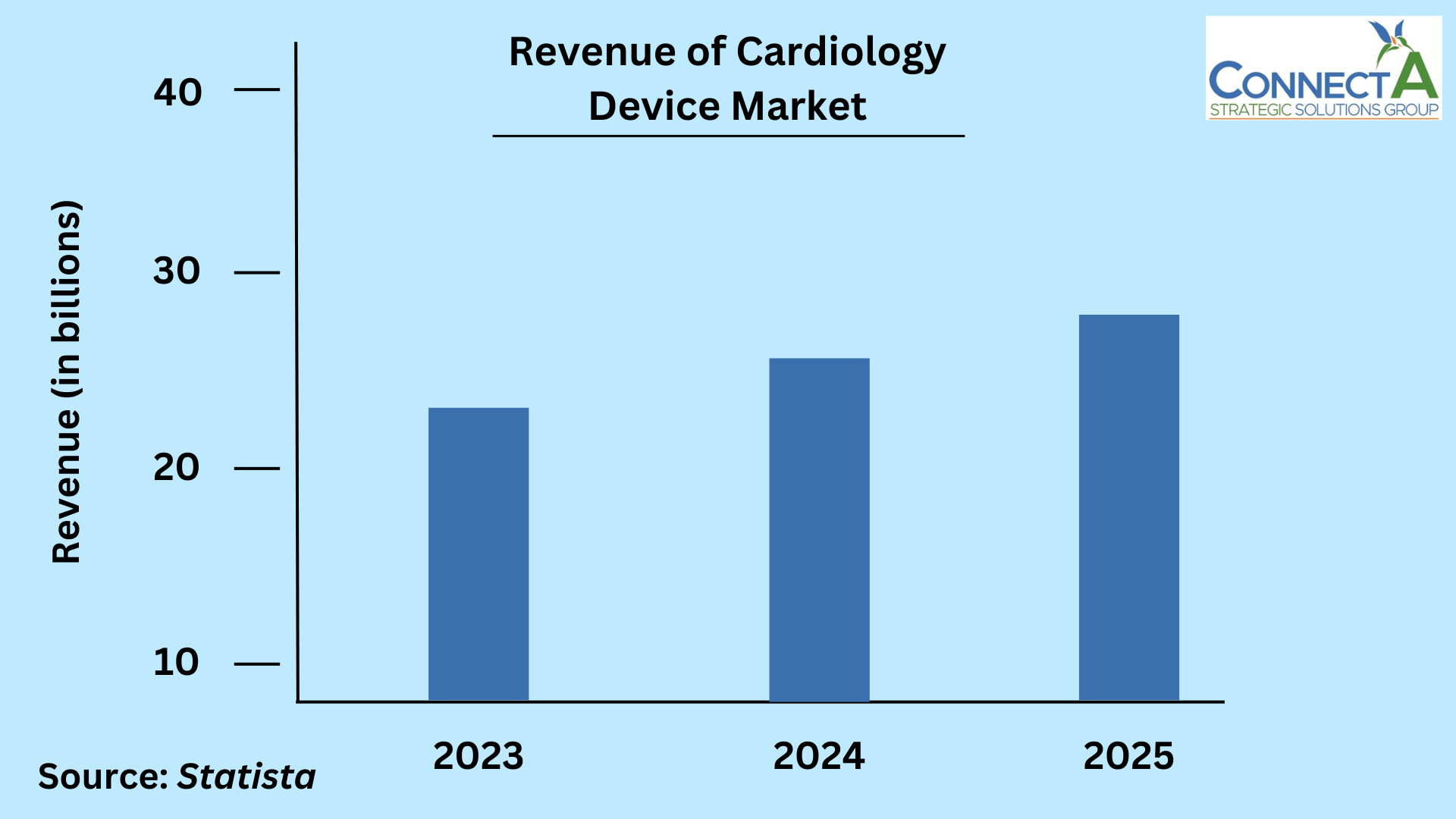
Your device’s messaging undoubtedly contributes to your GTM Strategy, and ultimately, your device’s success. However, it’s your market research that will provide valuable insights into the status of the medical device industry, which in turn will help determine your specific strategy. For instance, consider the industry’s Compound Annual Growth Rate (CAGR) of 5.9%, up to USD 799.67 billion4. Moreover, this growth in size can be seen in the Cardiology Device market, which has a market volume currently estimated at $25.77 billion in 2024, and is projected to reach $27.42 billion in 20255. The benefit of this comes into play with market shares. Companies with larger shares in a given market are comparatively larger to those with smaller ones. To put this into context, a 40% share is significantly larger than only a 20% share in a particular market6. Since large share companies typically have higher accrued sales within a given period of time, they in turn, are expected to have lower costs and higher profits6. However, for every device which makes it to market, there is one that’s recalled. There were approximately 34 (or 14.8% of recalls) medical device recalls between the years of 2017 and 20197. Though issued for various reasons, a lack of proper testing of products and services is a relatively common one.
While seemingly obvious, testing is an essential aspect of effective market research that is often forgotten. Neglecting to do so can result in a lack of understanding if said product meets what customers are expecting or requiring of the device8. Considering your product is intended to meet a specific market need, comprehensive testing and market analysis is essential to navigate current trends in the market, and receive feedback from users on product features and determine what may require improvement8. These insights will help as you differentiate yourself in comparison to your competitors, such as identifying a niche in your given industry, or having consistent branding8. Without understanding what sets you apart, there is no clear reason your target audience should pick your business over another.
As we discuss the intricacies of effective GTM Strategies, we must look at real world applications. One example of a medical device that struggled with its go-to-market strategy is the Proteus Digital Health "smart pill9." The product, designed to track medication adherence through an ingestible sensor, had an innovative concept but failed to gain widespread adoption. Its downfall was due to several strategic missteps. Though it gained FDA approval, Proteus faced difficulties convincing healthcare providers and investors of its value. The introduction of the pill sparked privacy concerns, with data collected through its sensor sent to a for-profit organization, as opposed to the patient's healthcare provider10. The device was also expensive and required complex integration into existing healthcare systems, which created barriers for both patients and practitioners11. Ultimately, these challenges, combined with a lack of clear market positioning and user adoption, led the company to file for bankruptcy, underscoring the importance of a well-executed go-to-market strategy in the medical device sector.
Overall, the role of effective messaging in a GTM Strategy cannot be overstated. It plays a pivotal role in how strategy as a whole is crafted. It's not just about the product itself, but how that product is communicated to your audience that makes the difference. Clear, compelling messaging ensures that your value proposition resonates with your target market, cuts through the noise, and distinguishes your offering from competitors. When done right, messaging aligns all aspects of your GTM strategy—from sales to marketing—and creates a cohesive, engaging narrative that drives brand loyalty, customer acquisition, and long-term growth. It’s the bridge between your product and your audience, making it indispensable to the success of any product launch or market entry.
Many need help with finding the right message for the right audience. This is where ConnectA Strategic Solutions Group comes in. If you need help launching your product to market, text Coaching to (908) 963-7747 or
click here to schedule a 30 minute strategy call.
1 - Product Marketing Alliance:
https://www.productmarketingalliance.com/your-guide-to-messaging/
2 - LinkedIn “The Five Pillar Got To Market Strategy”:
https://www.linkedin.com/pulse/five-pillar-go-to-market-strategy-stan-monlux/
3 - Kapstone Medical
4 - Yahoo Finance
https://finance.yahoo.com/news/medical-devices-market-size-share-124400001.html
5 - Statista
https://www.statista.com/outlook/hmo/medical-technology/medical-devices/united-states#revenue
6- Harvard Business Review
https://hbr.org/1975/01/market-share-a-key-to-profitability
7 - National Library of Medicine
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7395820/
8 - House of Revenue
https://www.houseofrevenue.com/blog/avoid-go-to-market-failure-6-key-areas-companies-struggle
9- Fierce Biotech
https://www.fiercebiotech.com/medtech/digital-pill-developer-proteus-files-for-bankruptcy
10 - National Library of Medicine
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9578571/#sec5
11 - Fierce Biotech
https://www.fiercebiotech.com/medtech/fda-elevates-philips-recall-hospital-home-ventilator-software